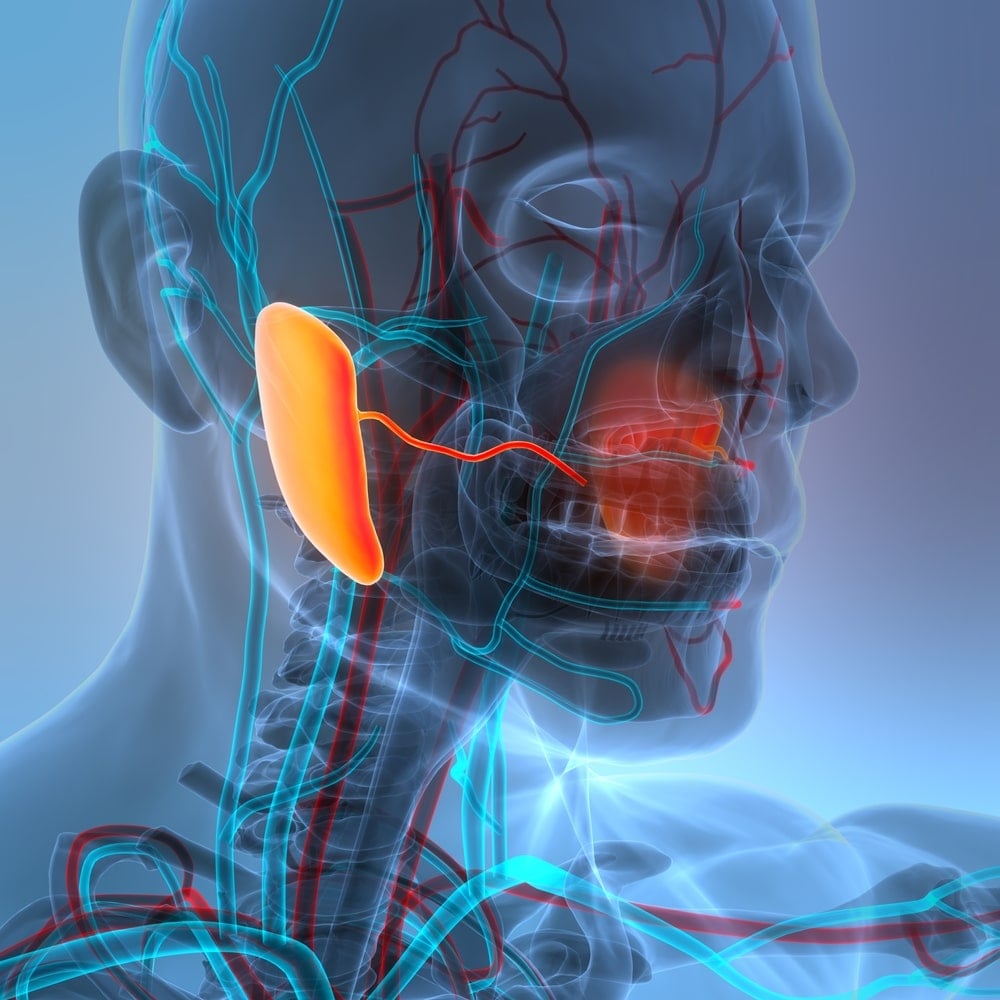
Doctors scanning cancer patients stumbled onto something nobody expected to find in 2020. Advanced imaging technology revealed structures that appeared on every single scan, glowing like beacons in a location where anatomy textbooks showed nothing macroscopic existed. Medical professionals had looked at this area behind the nose for centuries during examinations, surgeries, and dissections without recognizing what sat right in front of them. Students memorized anatomy atlases that mapped every organ, vessel, and tissue in the human body, yet these bilateral structures measuring roughly 4 centimeters each somehow remained invisible. When researchers confirmed their discovery through cadaver studies, questions emerged about how something this substantial stayed hidden and why it matters for patients receiving radiation treatment for head and neck cancers.
An Accidental Discovery During Cancer Scans
Prostate cancer patients routinely receive PSMA PET/CT scans to track their disease. PSMA stands for prostate-specific membrane antigen, a protein that appears on prostate cancer cells but also shows up in salivary gland tissue. Radiologists examining these scans noticed an unexpected pattern. Every scan showed bright uptake of the radioactive tracer in a specific area deep in the throat, behind the nasal passages.
At first, doctors assumed this represented normal variation or imaging artifacts. After reviewing dozens of scans, patterns became impossible to ignore. All patients showed identical bilateral structures in the same anatomical location. Intensity of the tracer uptake matched known major salivary glands, like the parotid and submandibular glands, under the jaw.
Researchers at the Netherlands Cancer Institute and the University Medical Center Utrecht decided to investigate systematically. They reviewed 100 consecutive PSMA PET/CT scans from patients with prostate or urethral gland cancer. Results proved striking. Every single patient, 99 males and one female, ranging from age 53 to 84, displayed clearly demarcated bilateral structures in the nasopharynx.
These structures extended from the skull base downward along the back and side walls of the throat. Length averaged 3.9 centimeters but ranged from 1 to 5.7 centimeters across patients. Total tracer uptake roughly equaled the sublingual glands under the tongue, significantly exceeding uptake in the palate, where minor salivary glands concentrate.

What Cadaver Studies Revealed
Seeing something on scans differs from confirming its physical existence. Researchers obtained tissue samples from the designated area in two human cadavers, one male and one female, donated to science. Dissection revealed large aggregates of predominantly mucous gland tissue exactly where imaging predicted.
Multiple macroscopically visible drainage ducts opened into the back and side walls of the throat. Gland tissue is draped over an anatomical landmark called the torus tubarius, a bulge formed by cartilage supporting the entrance to the auditory tube connecting the middle ear to the throat. Tissue extended downward along the pharyngeal wall and upward into a recess called Rosenmüller’s fossa.
Microscopic examination showed cells expressing PSMA almost uniformly throughout their cytoplasm, matching patterns seen in minor salivary glands. Cells produced mucous rather than watery saliva, similar to sublingual glands. Researchers found essentially no amylase expression, confirming very low numbers of serous acini that produce digestive enzymes.
Three-dimensional reconstruction using thin tissue sections revealed the spatial organization of glandular tissue and its drainage system. Multiple ducts converged from scattered gland lobules before opening into the throat. Structure resembled other salivary glands but with a distinct anatomical arrangement reflecting its location.
MRI imaging of a healthy volunteer showed subtle tissue with lower signal intensity on T2 sequences at the expected location on the inner side of the torus tubarius. Small bright dots within this tissue likely represented the macroscopic duct openings visible in cadavers.
Why Nobody Noticed Before
Human anatomy has been studied intensely for centuries through dissections, surgeries, and increasingly sophisticated imaging. Medical students memorize detailed atlases showing every structure from millimeter-sized nerves to major organs. How did bilateral structures averaging 4 centimeters each escape detection?
Several factors conspired to keep these glands hidden. Previous anatomical descriptions mentioned scattered acinar cell groups throughout the nasopharynx, but described them as dispersed rather than organized into distinct, clustered structures. Researchers knew about microscopic tubal glands lining the auditory tube itself, but considered them separate from any macroscopic gland.
Location proved crucial. These glands occupy a poorly accessible space under the skull base that can only be visualized through nasal endoscopy. Flat submucosal structures blend into the surrounding tissue without obvious boundaries during casual inspection. Duct openings visible during endoscopy were noticed but never interpreted as drainage from a larger unified gland.
Conventional imaging provided insufficient resolution and contrast. Ultrasound cannot penetrate bone to reach this area. CT scans show general anatomy but lack tissue specificity to distinguish the gland from the surrounding structures. Standard MRI sequences provide some information, but not enough to recognize salivary gland tissue definitively.
PSMA PET/CT changed everything. This molecular imaging technique combines the sensitivity and specificity needed to detect salivary gland cells with very high contrast compared to surrounding PSMA-negative tissues. Radioactive tracer binds specifically to PSMA-expressing cells, making salivary glands light up on scans while nearby muscle, fat, and connective tissue remain dark.
Naming the Discovery
Researchers proposed calling these structures tubarial glands based on their anatomical location, draping over the torus tubarius. This naming follows the pattern used for other major salivary glands. Parotid glands sit near the parotid duct. Submandibular glands occupy the submandibular space below the jaw. Sublingual glands rest under the tongue.
Alternative names included Eustachian glands or Rosenmüller’s glands after nearby anatomical landmarks. Researchers rejected these options because they did not precisely match the gland’s primary location. Medical terminology has also moved away from eponymous names honoring individual discoverers toward descriptive anatomical terms.
Debate emerged about whether tubular glands deserve classification as major salivary glands, minor salivary glands, or separate organs. Major glands typically have capsules enclosing discrete tissue masses. Minor glands exist as microscopic clusters scattered throughout the mouth and throat. Tubular glands share features of both categories.
Like major glands, tubular glands form macroscopically visible structures with organized drainage systems. Like minor glands, they lack complete capsules and are distributed along mucosal surfaces. They most closely resemble sublingual glands, which also contain predominantly mucous acini, show similar PSMA uptake, feature multiple drainage ducts, and include unencapsulated portions.
Some experts argued that all salivary glands form a continuum rather than discrete categories. Smaller and larger collections of acini together constitute a salivary gland system producing saliva throughout the mouth and throat. Under this view, tubarial glands represent newly recognized macroscopic components of this composite organ system.
Why This Matters for Cancer Patients
Discovery carries serious clinical implications for head and neck cancer treatment. Radiation therapy aims high-energy beams at tumors to kill cancer cells. Unfortunately, radiation damages healthy tissues in the beam path. Salivary glands prove especially vulnerable to radiation injury.
High doses cause interstitial fibrosis and acinar atrophy, destroying gland tissue and eliminating saliva production. Patients develop xerostomia, the medical term for dry mouth, along with dysphagia, meaning difficulty swallowing. Affected individuals struggle with food intake, digestion, and speech. Increased risk of dental cavities and oral infections compounds their problems.
Quality of life suffers tremendously. Imagine trying to eat crackers or bread with no saliva to moisten food. Speaking becomes difficult when your tongue sticks to the roof of your mouth. Taste perception changes. Constant thirst drives you to carry water bottles everywhere. Dental problems multiply despite meticulous hygiene because saliva normally protects teeth.
Radiation oncologists designate major salivary glands as organs at risk during treatment planning. Computer algorithms calculate radiation doses to tumors while attempting to spare surrounding healthy tissue. However, nobody included tubarial glands in these calculations because nobody knew they existed.
Researchers analyzed data from 723 head and neck cancer patients treated with radiation between 2007 and 2016. They retrospectively defined tubarial gland locations using anatomical landmarks derived from PSMA PET/CT scans and cadaver studies. The cranial border started at the skull base below the sphenoid sinus. The caudal border reached the level of the uvula base. Lateral borders followed the skull base above and fatty tissue below.
Statistical analysis revealed clear associations between radiation dose to the tubular glands and toxicity. Higher doses correlated with worse dry mouth and swallowing problems at 6, 12, 18, and 24 months after treatment. Effects remained statistically significant even after accounting for radiation to other known organs at risk, like parotid glands and swallowing muscles.
Every additional gray of radiation to the tubarial glands increased the risk of grade 2 or higher xerostomia and dysphagia. Grade 2 xerostomia means needing copious water or other lubricants with limitations to pureed and soft, moist foods. Grade 2 dysphagia means symptomatic swallowing problems requiring altered eating patterns.

Moving Forward With Treatment
Identifying tubarial glands as organs at risk opens opportunities to improve cancer treatment outcomes. Radiation oncologists can now include these structures in treatment planning algorithms. Sparing the tubarial glands while still delivering adequate tumor doses could reduce dry mouth and swallowing problems.
Challenges remain before widespread clinical implementation. Close proximity between the tubarial glands and the superior pharyngeal constrictor muscle complicates matters since both structures deserve protection. External validation using independent patient datasets needs to confirm these initial findings. Prospective studies should verify that delineation based on anatomical landmarks matches actual gland locations visible on PSMA PET/CT.
Changes to clinical protocols should occur only with continued monitoring of benefits. Radiation oncology combines science and art, balancing tumor control against normal tissue protection. Adding another organ at risk increases planning complexity and may require trade-offs with other structures.
Some anatomists questioned whether tubarial glands truly represent discoveries or simply a newly recognized clustering of previously described minor salivary glands. Historical literature mentions seromucous glands in this region, but as scattered microscopic structures rather than organized macroscopic entities. Debate about terminology and classification continues, yet clinical relevance seems clear regardless of how we label them.
My Personal RX on Supporting Salivary Health
Saliva plays more roles than most people appreciate until production drops. Beyond moistening food for swallowing and beginning starch digestion, saliva buffers acid, remineralizes teeth, controls bacterial growth, and enables taste perception. Discovering tubarial glands expands our understanding of this system’s complexity and highlights vulnerabilities during medical treatments. Even without radiation exposure, many factors impair salivary function. Common medications, including antihistamines, antidepressants, and blood pressure drugs, reduce saliva flow as side effects. Dehydration, mouth breathing, smoking, and aging all decrease production. Autoimmune conditions like Sjögren’s syndrome directly attack salivary glands. Supporting this system requires protecting glands from damage while optimizing conditions for healthy function. Saliva production depends on adequate hydration, proper mineral balance, and a healthy oral microbiome. Inflammation anywhere in the body can affect salivary glands, making systemic health approaches essential. People experiencing dry mouth often treat symptoms with artificial saliva substitutes without addressing root causes. Supporting natural saliva production through nutrition, hydration, and inflammation reduction offers better long-term solutions than constantly applying topical products.
- Maintain Optimal Hydration Throughout the Day: Dehydration directly reduces saliva production. Drink water consistently rather than large amounts occasionally. Electrolyte balance matters as much as volume, so include mineral-rich beverages or add trace mineral drops to water.
- Support Gut Health for Reduced Inflammation: Chronic inflammation impairs salivary gland function through multiple pathways. MindBiotic provides probiotics, prebiotics, and Ashwagandha KSM 66 to reduce inflammatory signaling throughout the body, including oral tissues.
- Choose Anti-Inflammatory Foods: Mindful Meals cookbook offers 100+ recipes emphasizing omega-3 fatty acids, polyphenols, and other compounds that reduce inflammation while providing nutrients needed for healthy gland function and saliva production.
- Stimulate Saliva Flow Naturally: Sugar-free gum or lozenges containing xylitol trigger mechanical stimulation of salivary glands. Xylitol also inhibits cavity-causing bacteria. Tart foods like lemon juice prompt immediate saliva release through reflex pathways.
- Avoid Mouth Breathing: Breathing through your mouth dries oral tissues and evaporates existing saliva. Address nasal congestion, sleep apnea, or habits causing mouth breathing. Nasal breathing maintains moisture and supports oral health.
- Limit Alcohol and Caffeine: Both substances have diuretic effects, promoting fluid loss and directly reducing saliva production. If consuming these beverages, increase water intake proportionally to compensate for fluid losses.
- Review Medications With Your Doctor: Many prescription and over-the-counter drugs list dry mouth as a side effect. Discuss alternatives with healthcare providers if medications significantly impair salivary function.
- Practice Oil Pulling: Swishing coconut or sesame oil for 10-15 minutes daily may support oral microbiome health and stimulate salivary glands through mechanical action, though research remains limited.
- Manage Stress Levels: Chronic stress reduces saliva production and alters its composition through hormonal mechanisms. Regular stress management practices support healthy salivary function alongside numerous other health benefits.
- Get Regular Dental Checkups: Reduced saliva flow increases cavity and gum disease risk. Professional monitoring and preventive care become even more important when salivary function declines for any reason.
Source:
Valstar, M. H., De Bakker, B. S., Steenbakkers, R. J., De Jong, K. H., Smit, L. A., Nulent, T. J. K., Van Es, R. J., Hofland, I., De Keizer, B., Jasperse, B., Balm, A. J., Van Der Schaaf, A., Langendijk, J. A., Smeele, L. E., & Vogel, W. V. (2020). The tubarial salivary glands: A potential new organ at risk for radiotherapy. Radiotherapy and Oncology, 154, 292–298. https://doi.org/10.1016/j.radonc.2020.09.034










 Subscribe to Ask Dr. Nandi YouTube Channel
Subscribe to Ask Dr. Nandi YouTube Channel









